
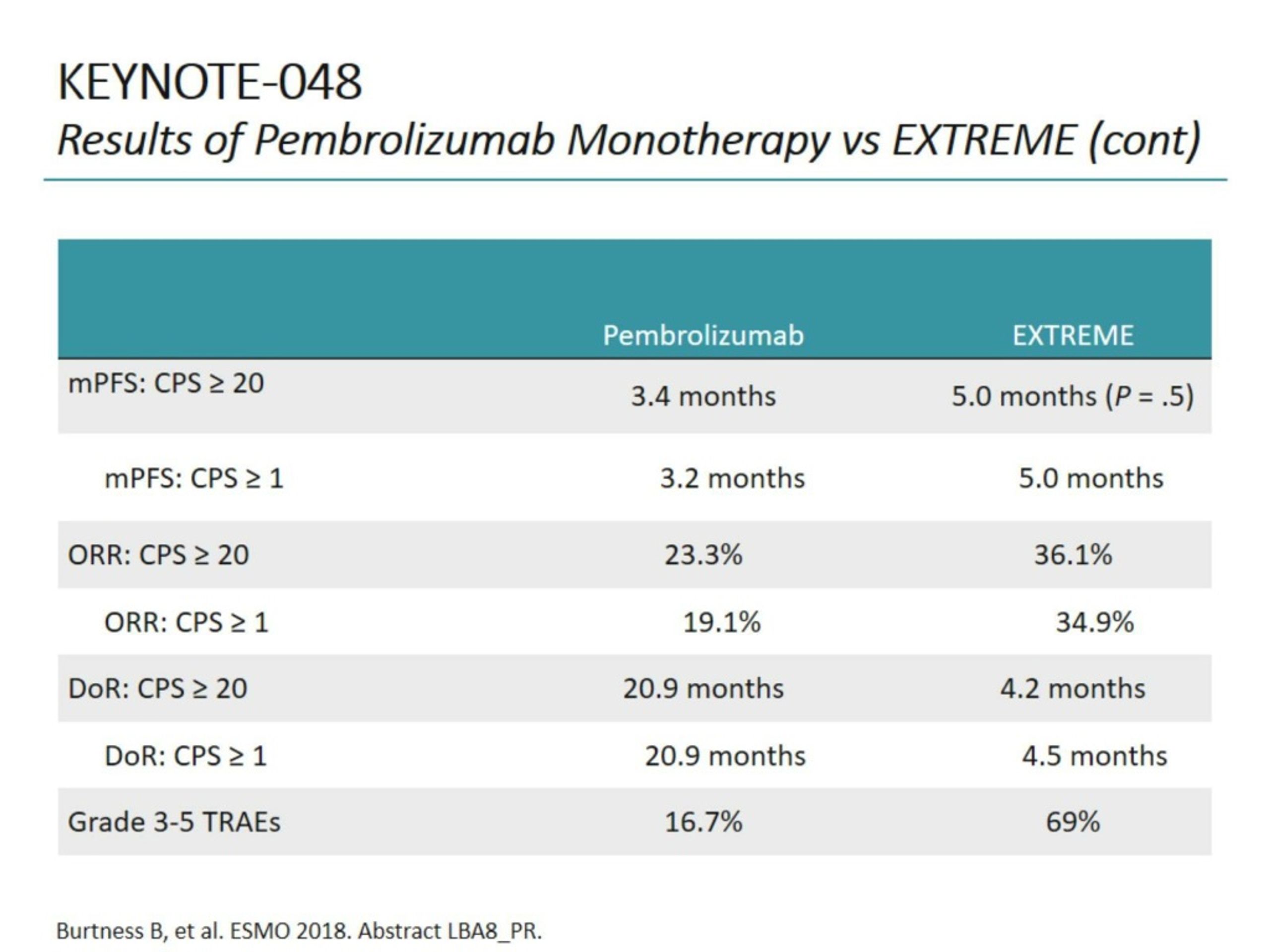
The objective response rate on second-course pembrolizumab was 27.3% (3 of 11). At data cutoff (February 18, 2020), overall survival improved with pembrolizumab in the PD-L1 CPS ≥ 20 (hazard ratio, 0.61 95% CI, 0.46 to 0.81) and CPS ≥ 1 populations (HR, 0.74 95% CI, 0.61 to 0.89) and was noninferior in the total population (HR, 0.81 95% CI, 0.68 to 0.97). The median study follow-up was 45.0 months (interquartile range, 41.0-49.2 n = 882). Efficacy was evaluated in programmed death ligand 1 (PD-L1) combined positive score (CPS) ≥ 20, CPS ≥ 1, and total populations, with no multiplicity or alpha adjustment. Patients were randomly assigned (1:1:1) to pembrolizumab, pembrolizumab-chemotherapy, or cetuximab-chemotherapy. Post hoc analysis of long-term efficacy and progression-free survival on next-line therapy (PFS2) is presented. Pembrolizumab and pembrolizumab-chemotherapy demonstrated efficacy in recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048.
17 Clinical Oncology Unit, Faculty of Medicine, University Malaya, Kuala Lumpur, Malaysia.16 University Hospital, Zurich, Switzerland.15 Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.14 Medical Oncology Department, Catalan Institut of Oncology - Badalona, B-ARGO Group, IGTP, Badalona, Spain.13 Royal Brisbane and Women's Hospital and University of Queensland, Brisbane, QLD, Australia.12 Medical University of Vienna, Vienna, Austria.11 Oslo University Hospital, Oslo, Norway.10 University of Kansas Medical Center, Kansas City, KS.9 Vall d'Hebron University Hospital, Vall d'Hebron Institute of Oncology, Barcelona, Spain.8 National and Kapodistrian University of Athens, Attikon University Hospital, Athens, Greece.7 Instituto do Cancer do Estado de Sao Paulo, Sao Paulo, Brazil.6 National Cancer Center Hospital East, Kashiwa, Japan.5 Centre Hospitalier de l'Université de Montréal, Montréal, QC, Canada.
4 Paracelsus Medical University Hospital, and Cancer Cluster Salzburg, Salzburg, Austria.3 Salzburg Cancer Research Institute-Center for Clinical Cancer and Immunology Trials, Salzburg, Austria.2 Yale Cancer Center and Yale School of Medicine, New Haven, CT.1 The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, National Institute of Health Research Biomedical Research Centre, London, United Kingdom.


 0 kommentar(er)
0 kommentar(er)
